Scientific Background
The Basics of Systemic Iron Homeostasis
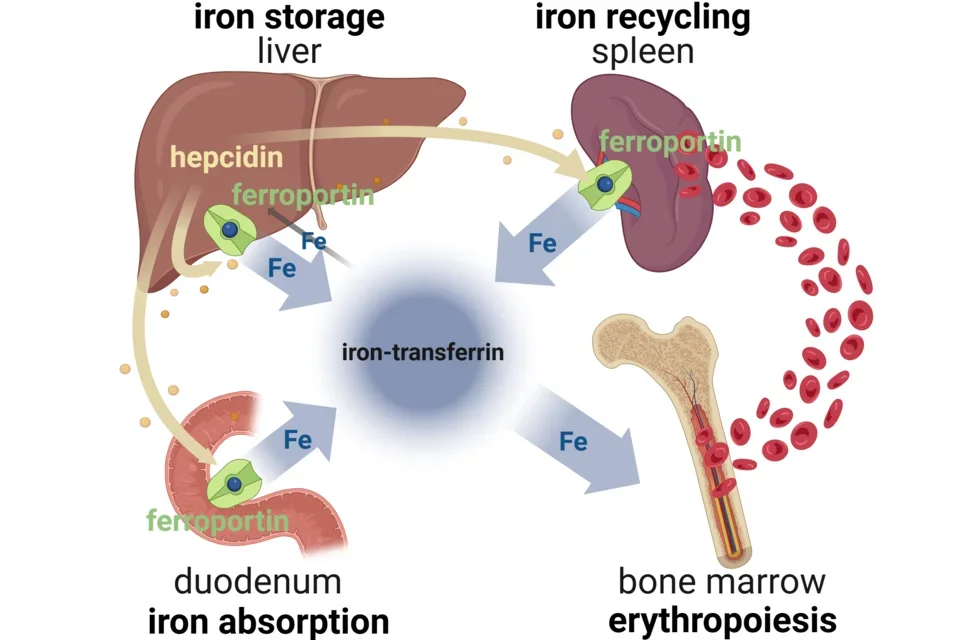
Ferroportin (also called SLC40A1) is the sole known cellular exporter of elemental iron. It mediates the delivery of iron to blood plasma from its three main sources:
- Dietary iron absorbed by duodenal enterocytes
- Recycled iron from macrophages that extract iron from senescent red blood cells
- Stored iron from hepatocytes
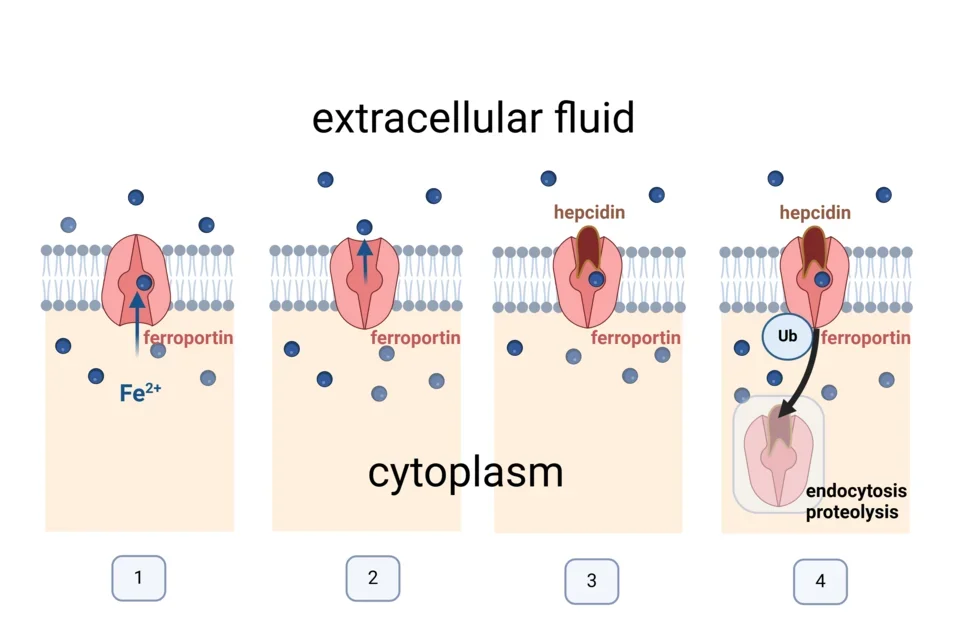
Ferroportin is a transmembrane protein which exports iron from cells in a highly regulated manner. Ferroportin is alternately open to the iron-rich cellular cytoplasm where it binds iron within its central cavity (1) and the relatively iron-poor extracellular fluid to which the bound iron is released (2). The ability of ferroportin to export iron is controlled by the iron-regulatory hormone hepcidin which binds to and occludes the open-out conformation of ferroportin (3). Ferroportin that binds hepcidin undergoes a conformational change followed by ubiquitination, endocytosis and proteolysis in lysosomes (4).
Ferroportin exports ferrous iron (Fe2+) which undergoes oxidation by a copper-containing ferroxidase to ferric iron (Fe3+) before binding to the plasma protein transferrin which distributes iron to tissues.
Plasma iron circulates bound to the iron-binding protein transferrin (iron-transferrin). Three main sources of iron contribute to the plasma iron pool:
- Dietary iron absorption predominantly in the duodenum
- The recycling of iron from red blood cells at the end of their lifespan (mainly in the spleen)
- Release of stored iron from hepatocytes (when needed)
Although all cells and tissues require iron, the main consumer of iron-transferrin is the bone marrow where iron is used by red cell precursors (erythroblasts), mainly for the synthesis of hemoglobin. The largest flow of iron is from red cell iron recycling to making new red cells in the marrow. Normally only about 5% of the iron available to tissues comes from iron absorption, and this iron balances the losses from the body through desquamation and minor blood losses.
The interaction of the liver-produced iron-regulatory hormone hepcidin with its receptor and iron exporter ferroportin regulates all the flows of iron into blood plasma.
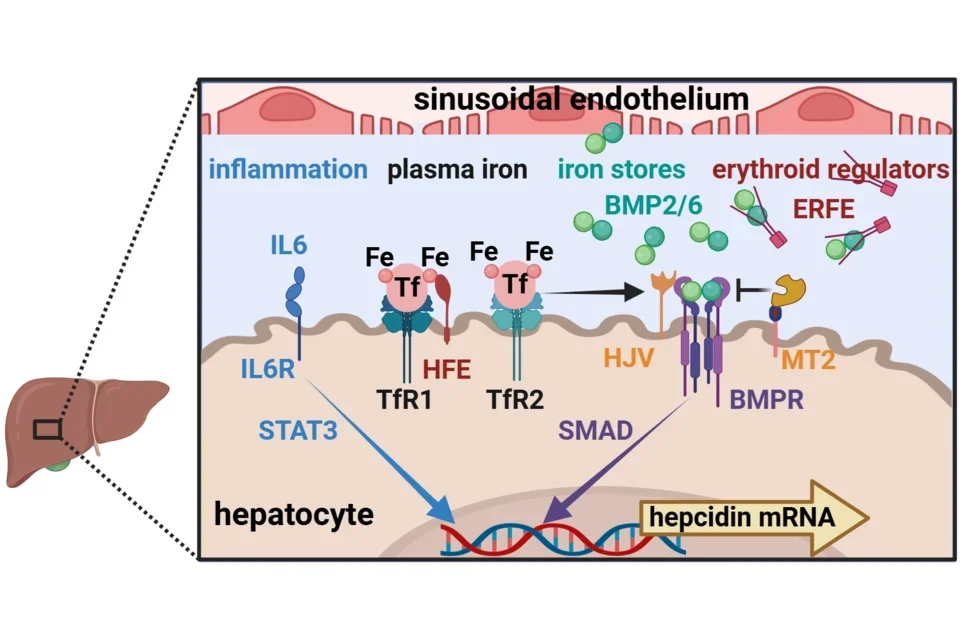
The production of hepcidin by hepatocytes accounts for nearly all of the hepcidin circulating in blood. However, regulation of hepcidin synthesis also requires another liver cell type, sinusoidal endothelial cells, located nearby. The synthesis of hepcidin is transcriptionally regulated by four main signals:
- Inflammation and host defense
- largely through the cytokine IL-6, its receptor IL6R and its JAK2-STAT3 signaling pathway
- Plasma concentration of iron-transferrin (Fe-Tf)
- by binding to transferrin receptors TfR1 and TfR2 on hepatocytes
- TfR1 and TfR2 are assisted by a membrane protein HFE in signaling through the bone morphogenetic protein receptor (BMPR)
- Iron stores in hepatocytes
- by regulating the production of bone morphogenetic proteins (BMP) 2 and 6 in sinusoidal endothelial cells
- Erythropoietic activity
- by erythroblasts secreting the erythroid hormone erythroferrone (ERFE) in proportion to the number of erythroblasts and their degree of stimulation by the erythropoietic hormone erythropoietin
The inflammatory pathway acts through its cognate binding sequences in the hepcidin promoter. The two iron regulatory pathways and the erythroid regulator converge on BMP receptors (BMPR), modified by iron-specific accessory membrane proteins hemojuvelin (HJV, a positive regulator) and matriptase-2 (MT2 also called TMPRSS6, a negative regulator). The BMP receptors signal through the SMAD pathway and its cognate binding motifs in the hepcidin promoter.